

01 Introduction to the study
Controlled switching of fluorophores between non-fluorescence and fluorescence states plays a key role in every super-resolution fluorescence microscopy technique, and exploring novel switching mechanisms remains critical to the performance of existing and emerging super-resolution methods. In this study, the authors propose a universal method for converting 3,6-diaminooxanthrone to a cage-free photoactivated fluorophore. Photoactivable xanthone ketone (PaX) efficiently and cleanly converts to highly fluorescent, photo- and chemically stable pyrroline dyes after light irradiation. At the same time, the strategy can also be extended to carbon- and silicon-bridged anthrone analogues, producing a series of photoactivatable tags sufficient to cover most of the visible spectrum. The results demonstrate the versatility and utility of PaX dyes in fixed- and live-cell labeling in conventional microscopy, as well as in the coordinate randomized and deterministic nanoobservation of STED, PALM, and MINFLUX.
Fluorescence nanomicroscopy enables minimally invasive observation of the internal structure and dynamics of molecularly specific biological samples with single-digit nanometer resolution, greatly improving our ability to observe (live) cells. At the heart of these techniques lies the intrinsic control between chemically specific fluorescent labeling and the on-off state of the fluorophore. Because switch-switching irreversible photoactivated dyes or cage dyes are used without the need for specific imaging buffers and high-intensity UV light, they are widely used in single-molecule localization microscopy. Photoactivated dyes are now used to reduce the fluorescent background in DNA coatings and to increase the number of cell structures that can be simultaneously imaged in stimulated emission loss (STED) microscopy that can be superimposed on channels. Among them, rhodamine dye has become one of the most used fluorophores in fluorescence microscopy and nanomicroscopy due to its optical and chemical stability, cell membrane permeability and significant tunable brightness, especially silicon rhodamine dye, which is favored for its excellent redshift emission, stable and durable fluorescence signal, good live-cell compatibility and brightness.
Prior to this, the caging strategy of rhodamine that has been reported has generally relied on "locking" the dye in a non-fluorescent form, i.e., by installing a light-resistant protective group (such as nitroquinofenoloxoxyl or nitroso) on the nitrogen atom, or by lactone ring synthesis to the corresponding cyclo α-diazoketone. However, both strategies have certain limitations, the former reduces the water solubility of the dye and produces potential toxic byproducts upon photoactivation, while the latter forms different non-fluorescent by-products, resulting in reduced main product abundance. Therefore, in fluorescence microscopy and nanotechnology, cage-free, compact photoactivated and biocompatible fluorophores are necessary if photoactivation is required to be performed quickly, completely, and without by-products.
02研究結果和討論
1. Synthetic design and mechanism of photoactivated labels
Here, the authors make a conjecture and verification of the reaction mechanism (Figure 1b), PaX after being illuminated by a wavelength of 405 nm, transitioning from the ground state to the excited state, and the lone pair of electrons on the additive still in the ground state acts to transfer electrons from the additive to PaX, and then form a new six-membered ring through electron transfer on the olefin, and finally protonation to form a closed PaX-CF after electron transfer in the ring.

Figure 1: Design, synthesis, and characterization of PaX dyes
The authors employ a photo-induced assembly ("locking") method that relies on fluorophores, whereas photoactivated dyes used in nanomirrors typically rely on the release ("unlocking") of cage-like groups.
b. General structure of PaX with 1-alkenyl radical trap and its 9-alkoxypyridine photoproduct (closed form, CF), and the proposed photoactivation mechanism.
c. Synthetic route for preparing PaX.
Absorption and fluorescence spectra of compound 1 (1.66 μg ml-1) over time in phosphate buffer (100 mM, pH 7; λact = 405 nm).
Under the same conditions as d, compare the photoactivation kinetics of silicon bridge PaX 1–6.
Under the same conditions as d, the photoactivation kinetics of PaX dye 9-12 were compared.
Compare the photoactivation kinetics of compound 11 (3.8 μM) in phosphate buffer (100 mM) at different pH values (λact = 405 nm).
Measure the photofatigue resistance of 11-CF and established commercial fluorophores with similar spectral properties in phosphate buffer (λexc = 530 nm).
To study the effects of radical receptor substitution, the authors synthesized a series of photoactivatable Si-xanthones (1-7; Figure 1c). The product of interest was prepared by iridium-catalyzed, chelation-assisted, ortho-selective diarylketone C-H boronation reaction (A). Under the action of KF, the generated borate ester (B) is converted to the corresponding aryl bromide (C) by the CuBr2-pyridine system, and finally a series of olefins are substituted under standard Suzuki-Miyaura cross-coupling conditions.
2. Photoactivation tags without cage-like groups for nanomirrors
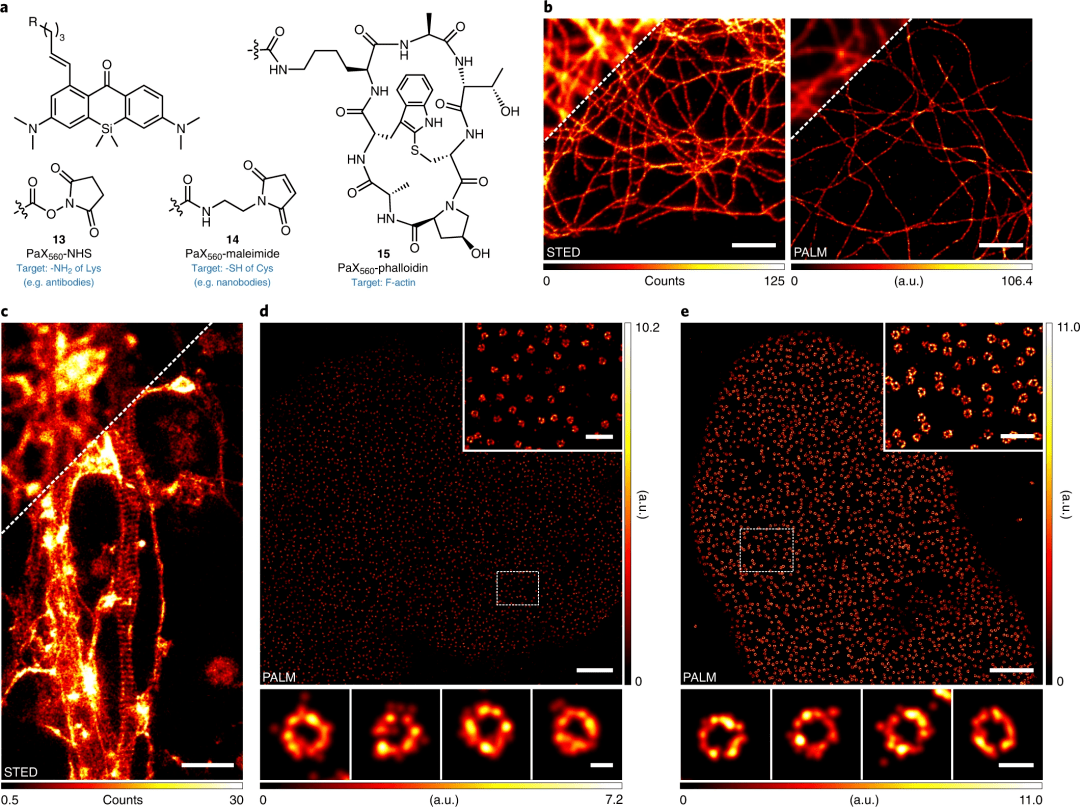
Figure 2: Light-activated tags for optical nanomirrors
Structure of PaX560 derivatives for bioconjugation (13, 14) and actin-labeled (15).
STED (left) and PALM (right) images of microtubules in COS-7 cells, labeled with indirect immunofluorescence labeling with secondary antibodies with 13.
Actin structure of periodic membrane cytoskeleton in fixed primary hippocampal neuronal culture axons, labeled with 15 and placed in Mowiol.
PALM image of NPCs in COS-7 cells by indirect immunofluorescence, with anti-NUP98 primary antibody and with 14-labeled secondary nanobodies.
PALM image of NPCs in HeLa-Kyoto cells expressing NUP107-mEGFP, labeled with anti-GFP nanobodies conjugated to 14.
Scale bars: 2 μm (b-e, main image), 500 nm (d, e inset), 50 nm (d, bottom), 100 nm (e, bottom).
3. Targeted tags for live-cell imaging
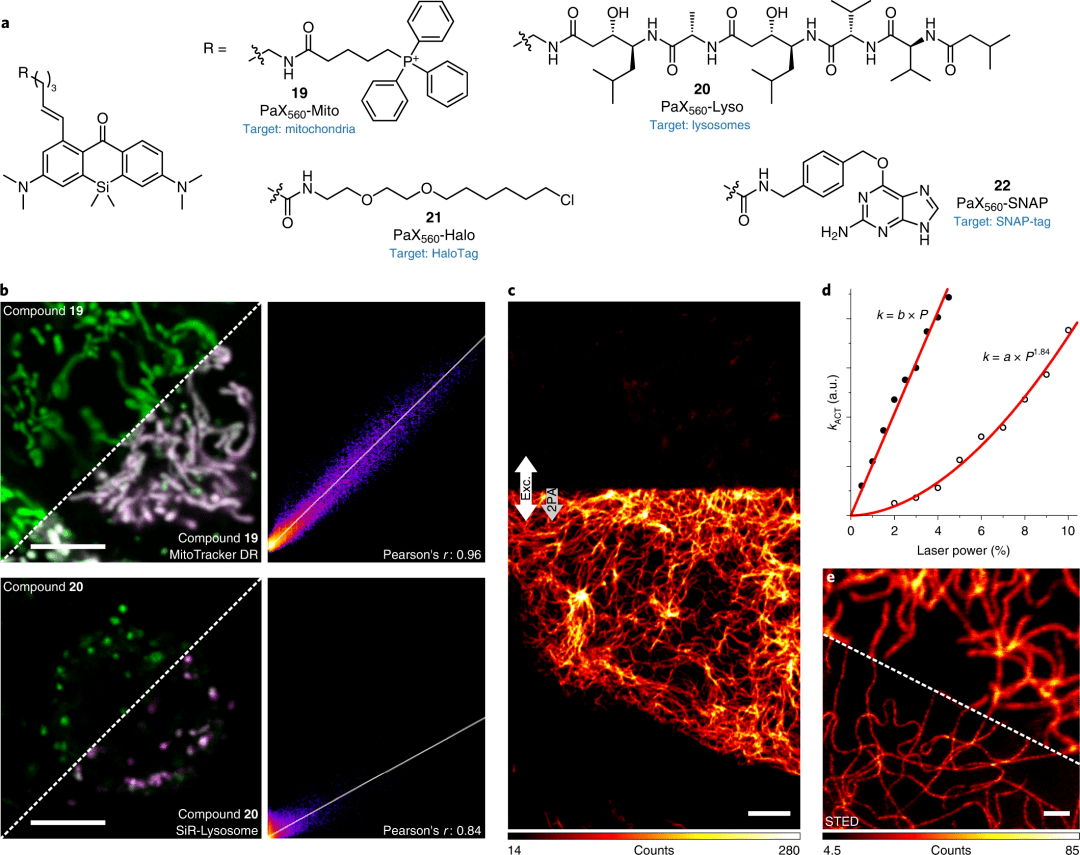
Figure 3: Imaging using photoactivatable PaX tags in living cells
Structure of PaX560 derivatives (19-22) for live-cell imaging.
Confocal images of COS-7 cells co-incubated with 19 (200 nM) and MitoTracker Deep Red (50 nM, upper layer) or 20 (20 nM) and SiR-lysosomes (200 nM, lower layer) and corresponding Pearson correlation analysis). Conversion to 19-CF and 20-CF was achieved using a 355 nm laser.
Confocal images of wave protein filaments labeled 21 (200 nM) before activation (upper layer) and two-photon activation (2PA) (lower layer, indicated by arrow) in U2OS cells.
d. Diagram of the activation rate and laser power of a single-photon (355 nm, 0.3 μW at 100%) or two-photon activated laser (810 nm, 109 mW at 10%).
Confocal (top) and STED (bottom) images of the same sample after laser activation at 405 nm.
Scale bars: 5 μm (b, c) and 1 μm (e).
4. Multiplexed PaX tags are activated by selective light
Given the difference in photoactivation rates of PaX dyes, the authors speculate that two complementary tags can be used for multiplexing, sequentially applying lower and higher doses of activated light, first converting a fluorophore (e.g., PaX560) while retaining the more difficult-to-activate fluorophore (e.g., PaX480) until a higher photodose is applied. First the authors tested this by confocal imaging, then in fixed cells by two-color single detector PALM imaging, labeled with organelles (Pax560–Mito 19 or Pax560–Lyso 20) and Halotag specificity (Pax480–Halo, 23), further demonstrating sequential activation in living cells by confocal imaging.
03研究結論
The authors introduce a universal design strategy for cage-free, bright, and live-cell-compatible photoactivated dyes suitable for a wide range of light microscopy and nanotechnologies, including PALM, STED, and MINFLUX. The unique structural features of these PaX dyes functionalize the photoresponsive 3,6-diaminoxanthone core with the intramolecular olefin radical trap, providing highly compact and inherently uncharged, intact cell membrane permeable labeling. Under single- or two-photon activation, these compounds rapidly form highly photostable fluorescent pyrroline dyes. By changing the substituents of the PaX dye, the photoactivation kinetics as well as spectral properties can be changed, enabling multiplexed pseudo-color and traditional multicolor imaging.
The utility and versatility of PaX dyes in fixed and live-cell super-resolution fluorescence microscopy experiments has been demonstrated by a variety of target-specific probes and labeling strategies. This approach will further stimulate the development of photoactivated probes and sensors for bioimaging and materials science.
(More research interpretations will be shared later)
04超高分辨率顯微成像系統(tǒng)iSTORM
iSTORM, the ultra-high-resolution microscopic imaging system released by Ningbo Lixian Intelligent Technology Co., Ltd. (INVIEW), adopts STORM super-resolution microscopy imaging technology derived from the principle of the Nobel Prize in Chemistry, and realizes the breakthrough of the diffraction limit of optical microscopy, making the research on single-molecule localization and counting of biological macromolecules, subcellular and supramolecular structure analysis, and biodynamics of biological macromolecules on the resolution scale of 20 nm become a reality, so as to provide a reality for life sciences, Major breakthroughs in fields such as medicine.

Figure 4: iSTORM, an ultra-high-resolution microscopic imaging system independently developed by Lixian Intelligence.
Ultra-high resolution microscopy imaging system iSTORM has the characteristics of 20 nm ultra-high resolution, 3-channel simultaneous imaging, 3D simultaneous shooting, real-time reconstruction, and 2-hour novice mastery, etc., has realized the localization and counting of single molecules in living cells, and provides fluorescent dye selection, sample preparation, imaging services and experimental solutions as an overall solution. It has been highly recognized by more than 50 scientific research groups and more than 100 researchers.
References:
Lincoln, R., Bossi, M.L., Remmel, M. et al. A general design of caging-group-free photoactivatable fluorophores for live-cell nanoscopy. Nat. Chem. (2022).


